Abstract
Introduction and Rationale:
The development of alloantibodies to factor VIII remains a challenging and significant complication in the treatment of patients with congenital hemophilia A (HA). Inhibitor development is more common in severe HA than in non-severe HA (NSHA). Incidence of inhibitors in NSHA is approximately 3-13%, and even with attempted immune tolerance induction and immunosuppression, inhibitors have been reported to persist long-term in about 40% of NSHA patients (Hay 1998). Inhibitors in patients with NSHA can have variable kinetics, causing clinical characteristics similar to patients with acquired hemophilia A (AHA), with observable type 2 kinetics and inhibition of exogenous and endogenous FVIII activity (d'Oiron et al. 2006). Optimal treatment regimens have yet to be established for these patients, in terms of inhibitor eradication and choice of agent for control of bleeding episodes, with known thrombotic side effects when using bypassing agents, including in pediatric patients (d'Oiron et al. 2006).
It has been observed as well that the efficacy of bypassing agents, such as activated prothrombin complex concentrates (aPCC) and recombinant activated factor VII (rFVIII), is diminished in the presence of an inhibitor (Rocino et al. 2017). Challenge with aPCC or rFVIII can also induce inhibitor recurrence in patients who have had previous resolution (Giuffrida et al. 2008).
Recombinant porcine FVIII (rpFVIII) is an alternative therapy, which by virtue of its incomplete homology in the A2 and C2 domains of the FVIII molecule, has decreased reactivity with anti-human FVIII (hFVIII) inhibitors (Mulliez et al. 2014). rpFVIII has demonstrated efficacy in patients with severe HA with inhibitors, as well as in acquired HA (AHA) (Kempton et al. 2012; Kruse-Jarres et al. 2015). However, experience is limited for use in patients with NSHA and inhibitors. We report successful bleed treatment with rpFVIII of 3 adult patients and 1 child with NSHA who developed inhibitors at the University of North Carolina, with excellent hemostatic efficacy and no adverse treatment-related effects.
Methods:
We analyzed clinical, pharmacy, and laboratory data from 4 patients with NSHA who developed inhibitors treated with rpFVIII after failure of or contraindication to bypassing agents at the University of North Carolina. Investigational review board approval was obtained for our data collection and analysis.
Results:
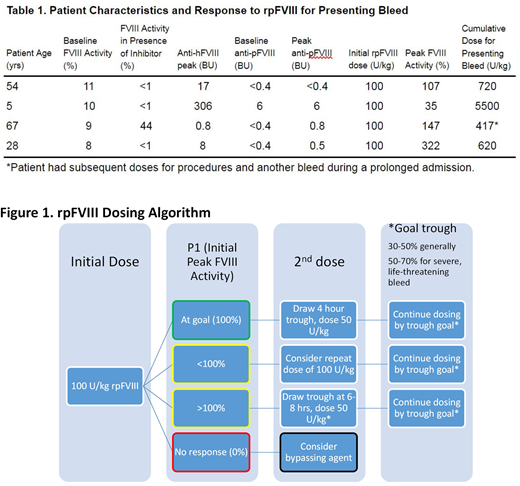
Average baseline FVIII activity was 9.5%, with 3 of our 4 patients having a FVIII activity of <1 after inhibitor development. Mean anti-human FVIII inhibitor titer (hBIA) was 83 BU, ranging from 0.8 to 306 BU. Mean cumulative dose of rpFVIII used in the first 24 hours was 735 U/kg, but only 163 U/kg in our adult patients. Mean number of infusions used in the first 24 hours was 2.3 in our adult patients, and the pediatric patient required 18 infusions in the first 24 hours. Mean peak FVIII recovery was 153%. Hemostasis was achieved in all 4 patients with no treatment-related adverse effects, despite 3 of 4 patients developing a detectable anti-porcine FVIII inhibitor titer (pBIA).
Conclusions:
rpFVIII appears to be a safe and effective therapy in patients with NSHA who develop inhibitors. Our patients treated with immunosuppression in addition to rpFVIII, similar to how we treat patients with AHA. Of note, we achieved effective hemostasis in our 3 adult patients at much lower than FDA-recommended doses for AHA by using our previously established dosing algorithm for rp
FVIII (Martin et al. 2016; Fig 1). Our pediatric patient required much more rpFVIII for effective hemostasis. This patient also had a much higher hBIA, and was being treated after the failure of multiple bypassing agents. However, once it seemed inhibitor binding sites were saturated after frequent higher rpFVIII doses, FVIII recovery was achieved, with concomitant decrease in doses required. FVIII activity was an effective means of monitoring efficacy of rpFVIII use, as in severe HA and AHA. One adult patient in particular had a very good clinical response to rpFVIII, requiring only 1 dose in the first 24 hours, with doses as low as 19 U/kg given daily required for bleeding control.
Abajas:CSL Limited: Honoraria; Bayer: Honoraria. Moll:Stago Diagnostics: Consultancy. Ma:CSL: Consultancy; Novo Nordisk: Consultancy; Shire: Honoraria, Research Funding; CVS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal